Japan approves Alzheimer's drug developed by Eisai, Biogen
TOKYO - Japan's health ministry on Monday approved the manufacture and sale of a drug for Alzheimer's disease developed by Japanese pharmaceutical company Eisai Co. and US firm Biogen Inc., paving the way for the drug to be made available for use within the year.
Lecanemab, branded Leqembi, will be the first drug in Japan to both treat the underlying cause of the debilitating neurodegenerative disease and slow the progression of its symptoms, reported Kyodo News.
"We have turned a new page in the history of Alzheimer's disease treatment," Eisai CEO Haruo Naito said in a statement.
Citing the disease not only causes significant impairment and burden for the people living with it and their caretakers, Naito said, "We are committed to delivering Leqembi...as a new treatment that removes the cause of the disease," benefiting patients and their families.
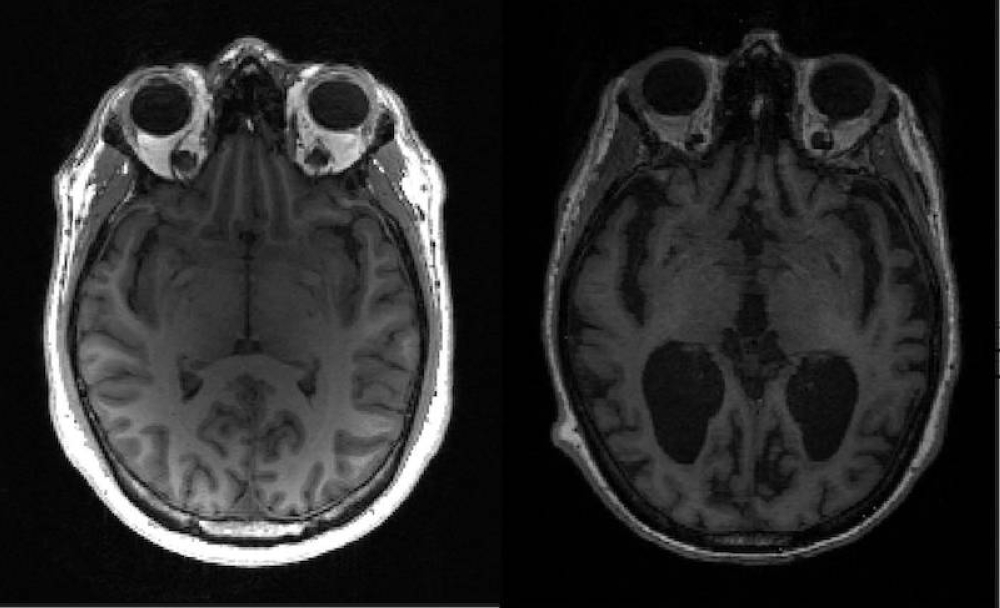
The drug, which is for treating early-stage Alzheimer's and mild cognitive impairment, is a special type of antibody that targets a protein called amyloid beta. The plaque-like protein, which accumulates inside the brain and destroys nerve cells, is considered the cause of the disease.
The drug is administered via an intravenous injection every two weeks. Eisai projects that about 1 per cent of the estimated 5 million to 6 million with the specific health conditions it targets would actually use it.
The move came after US regulators formally approved lecanemab in July after granting the drug a fast-track conditional approval in early January. In Japan, a health ministry panel gave the green light to its formal approval in late August.
Eisai has applied for approval of the drug in other countries, including China, Canada and Britain.
As the new drug's standard price is US$26,500 per year in the United States, the price in Japan is also likely to be high.
Eisai said clinical trials demonstrated the drug curbed the progression of such symptoms as memory loss and impaired judgment by 27 percent compared with placebo.
However, some patients administered the drug experienced side effects like brain edema and bleeding, it said. - BERNAMA
Download Sinar Daily application.Click Here!














